Introduction: Sickle Cell Disease (SCD) is an inherited blood disorder with profound implications on a wide variety of tissue types (Kavanagh et al., 2022). As many as 37% of patients with SCD experience silent cerebral infarction by age 14 (DeBaun et al., 2012). Neurological insults can increase risk for voice and swallow disorders which compromise physical health and diminish quality of life for many individuals (Aronson & Bless, 2009). In fact, voice disorders (dysphonia) affect 6% of adults (Roy et al., 2005), and swallowing difficulties (dysphagia) impact as many as 16% (Adkins et al., 2020). Since both phonation and deglutition require the coordination of multiple muscles and nerves (Kendall, 2008), diseases such as SCD may be associated with elevated risk for dysphonia and dysphagia. Unfortunately, the frequency of these symptoms in individuals with SCD has rarely been considered. This study examined the prevalence and nature of dysphonia and dysphagia in individuals with SCD.
Methods: Adults (age >18) diagnosed with SCD were surveyed regarding the presence of voice and swallow complaints, symptomology, voice-use patterns, voice and swallow-related quality of life, demographic information, and medical history. Additionally, participants completed the Voice Handicap Index-10 (VHI-10), Eating Assessment Tool (EAT-10), and Reflux Severity Index (RSI). To enable comparisons with previous epidemiological studies of voice and swallow disorder prevalence, survey questions were adapted from previously published investigations (Roy et al., 2004, 2005). Participants were recruited from an outpatient hematology clinic during routine visits and completed the survey online using Redcap. Univariate and multivariate analyses were used to identify dysphonia and dysphagia risk.
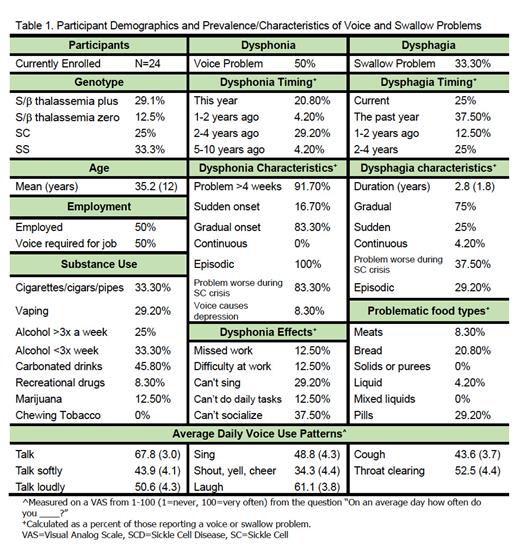
Results: Twenty-four patients have completed this study (males=16, females=8; mean age=35.2 years; genotype: SS=8, S/β thalassemia zero=3, SC=6, S/β thalassemia plus=7) with recruitment ongoing. Forty-five percent of patients reported a voice complaint within the last 4 years with symptoms lasting longer than 4 weeks in all cases (Table 1). Sixty-six percent of patients presented with VHI scores indicating a voice problem. Dysphonia was described as episodic, with 83.3% of cases becoming more severe during sickle cell crises. Voice problems resulted in exclusion from social activities (37.5%), missed days of work (12.5%), inability to perform daily tasks (12.5%), inability to sing (27.2%), and difficulty performing one's occupation (12.5%). Individuals who vaped were 8% more likely to report dysphonia ( p=.04), and those who drank more than 3 alcoholic beverages a week were 25% more likely to report dysphonia ( p=.03). Patients with dysphonia presented with significantly more severe VHI scores ( p=.019, dysphonia=15.1, normophonic=10.0) and RSI scores ( p=.036, dysphonia=10.5, normophonic=6.8) than those with no voice complaint. No voice use pattern predicted voice disorder risk.
One third of participants reported a swallow complaint, with 75% of cases occurring within the last 2 years (Table 1). Forty-one percent of patients presented with EAT-10 scores indicating dysphagia. Swallowing problems were evident with meats (8.3%), bread-like consistencies (20.8%), pills (29.2%), and thin liquids (4.2%). Dysphagia symptoms were episodic in 87.5% of cases, becoming more severe during sickle cell crisis in 37.5% of cases. Patients with dysphagia symptoms presented with significantly more severe EAT-10 scores than those without swallow symptoms ( p<.001, dysphagia group=6.12, no dysphagia=2.06). No patients reported seeing a medical professional for dysphonia or dysphagia symptoms.
Conclusions: Dysphonia and dysphagia were markedly elevated in patients with SCD when compared with the general population. Although these symptoms diminished quality of life, no patient had received treatment or even evaluation of these symptoms. Given the medical complexity of patients with SCD, diagnostic overshadowing might have precluded thorough workups of symptoms. This is unfortunate as impairments in voice and swallow function greatly impact quality of life and, in some cases, can be life-threatening. If the current findings are confirmed by future large-scale studies, individuals with SCD may benefit from proactive systematic monitoring and treatment of the subsystems responsible for voice and swallow.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal